I've seen too many expensive CNC parts get rejected because engineers didn't understand anodizing basics. Getting the surface finish wrong can ruin weeks of precision machining work.
Anodizing is an electrochemical process that creates a protective oxide layer on metal surfaces. This controlled oxidation enhances corrosion resistance, improves durability, and allows for coloring while maintaining dimensional stability. The process works particularly well with aluminum, transforming its surface into a hard, wear-resistant coating.
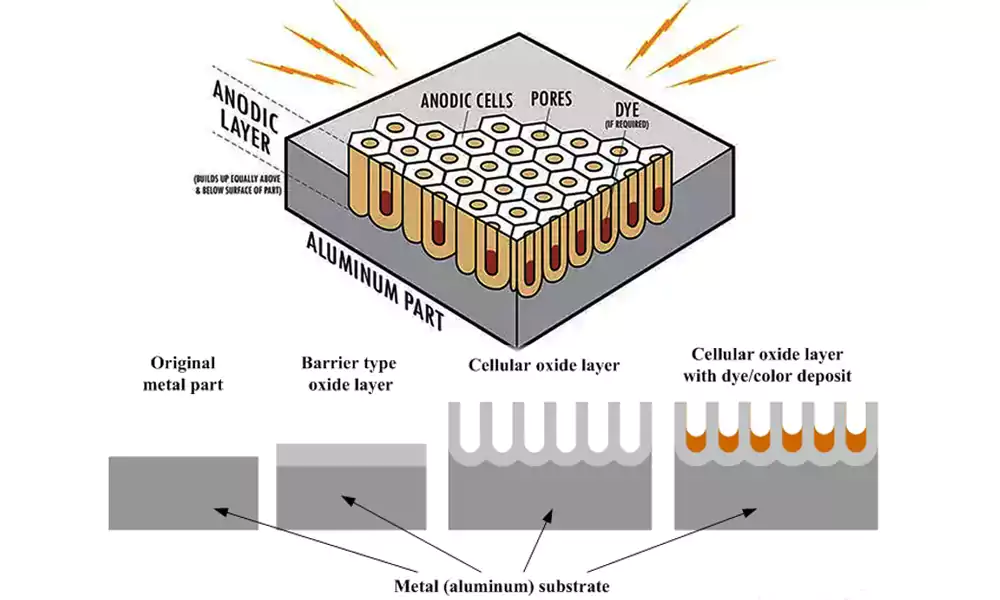
Many engineers think anodizing is just about adding color to parts. But in my fifteen years running Allied Metal, I've learned it's actually about engineering the surface properties. Let me show you how to get the most from this essential manufacturing process.
What is Anodizing?
Have you ever wondered why aluminum aircraft parts don't corrode despite constant exposure to harsh environments? The secret lies in understanding what anodizing really accomplishes.
Anodizing is an electrochemical process that thickens aluminum's natural oxide layer. Unlike paint or plating, anodizing converts the metal surface into a decorative, durable coating that's integral to the substrate. The resulting finish won't peel or chip under normal conditions because it's part of the metal itself.
The Science Behind Surface Transformation
The fundamental principle is straightforward: we're accelerating and controlling nature's corrosion process. Aluminum naturally forms a thin oxide layer when exposed to air, but anodizing makes this layer hundreds of times thicker and more durable. The process creates a porous aluminum oxide1 structure that can be sealed for protection or colored with dyes for aesthetics.
Key Characteristics of Anodized Surfaces
What makes anodizing unique is its integration with the base material. Since the coating grows from the original metal, it provides exceptional adhesion that applied coatings can't match. The hardness achieved through hard anodizing can reach 60-70 Rockwell C, making it suitable for high-wear applications. The electrical insulation properties also make it valuable for electronic components.
What is the Purpose of Anodizing?
Why would you choose anodizing over simpler painting or powder coating? The answer becomes clear when you understand the multiple engineering benefits beyond mere appearance.
Anodizing serves multiple purposes: it dramatically improves corrosion resistance, enhances surface hardness, provides electrical insulation, and allows for permanent coloring. The anodized layer also serves as an excellent base for adhesive bonding and improves paint adhesion when needed.

Enhancing Functional Performance
Beyond cosmetics, anodizing solves real engineering challenges. I recently worked with a medical device company that needed aluminum components to withstand repeated sterilization without degrading. Anodizing provided the perfect solution - the hard surface resists scratching during handling, while the sealed pores prevent bacterial growth. For electronic enclosures, anodizing offers electrical insulation that powder coating can't match.
Extending Product Lifespan
The economic benefits are substantial. Properly anodized components can last decades in harsh environments. We've seen anodized architectural aluminum maintain its appearance after 30 years of weather exposure. The maintenance-free nature of anodized surfaces makes them cost-effective for applications where recoating would be difficult or expensive.
How Does Anodizing Work?
Understanding the step-by-step process helps explain why anodizing delivers such consistent, reliable results across production runs.
Anodizing works by making aluminum the anode in an electrochemical cell. When current flows through sulfuric acid electrolyte, oxygen generated at the aluminum surface reacts with the metal to form aluminum oxide. This creates a porous coating that can absorb dyes before being sealed for permanence.
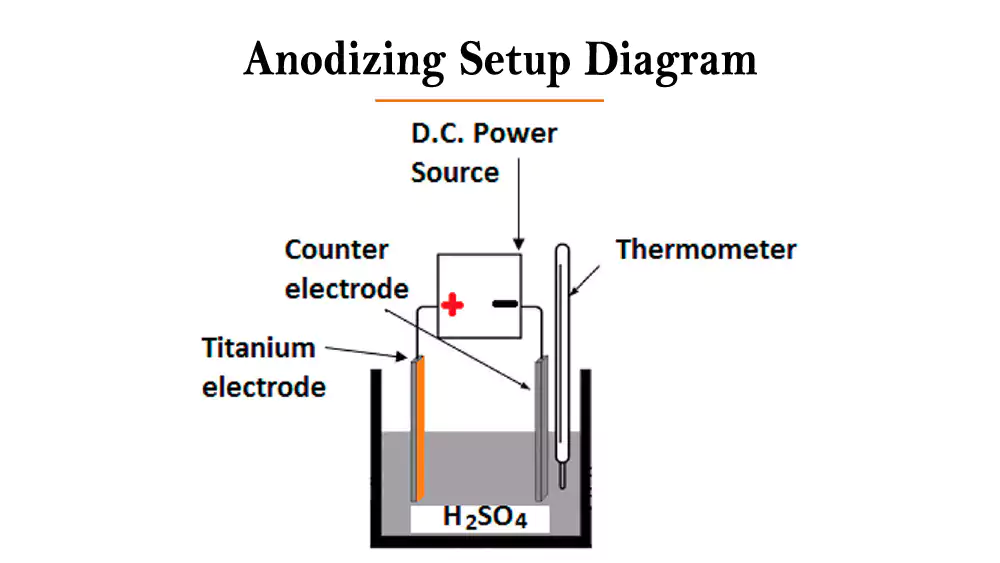
The Seven-Step Manufacturing Process
The journey from raw machined part to finished anodized component involves precise stages:
- Cleaning: Removing machining oils, fingerprints, and contaminants
- Etching: Creating a uniform matte surface texture
- Desmutting: Removing alloying element residues
- Anodizing: Building the oxide layer through electrolysis
- Coloring: Introducing dyes into the porous structure
- Sealing: Closing the pores to lock in color and properties
- Quality Verification2: Testing thickness, color, and corrosion resistance
Critical Process Controls
Each stage requires precise control. Temperature variations of just 5°F can affect coating hardness by 20%. Current density3 must be maintained within tight limits to ensure uniform thickness. The quality of rinsing between stages prevents contamination that could cause spotting or poor adhesion.
Anodizing Applications for Common Metal Materials
Not all metals respond equally to anodizing processes. Understanding material compatibility prevents costly mistakes in product design.
Aluminum and its alloys are ideal for anodizing, with titanium and magnesium also responding well. Steel, copper, and zinc cannot be anodized using conventional methods and require alternative surface treatments for similar benefits.
Aluminum Alloys and Their Characteristics
| Alloy Series | Anodizing Response | Typical Applications | Special Considerations |
|---|---|---|---|
| 1000 Series | Excellent | Decorative trim, reflectors | Pure aluminum anodizes clearly |
| 2000 Series | Good (Type II) | Aerospace components | Copper content may darken finish |
| 5000 Series | Very Good | Marine hardware, automotive | Magnesium provides good corrosion resistance |
| 6000 Series | Excellent | Architectural, consumer products | Balanced properties for most applications |
| 7000 Series | Good | High-strength applications | Zinc may cause darker appearance |
Non-Aluminum Metals and Alternatives
While aluminum dominates anodizing applications, titanium anodizing creates beautiful interference colors without dyes, popular in aerospace and medical applications. Magnesium anodizing provides corrosion protection4 for lightweight applications. For ferrous metals, alternative processes like phosphating or black oxide provide similar corrosion protection with different aesthetic results.
Anodizing Process for Aluminum Alloys
Material selection dramatically impacts anodizing results. Choosing the right aluminum alloy ensures both mechanical performance and surface finish quality.
Different aluminum alloys require specific anodizing approaches due to varying silicon, copper, and magnesium content. 6061 aluminum produces excellent gray finishes, while 2024 may yield darker tones. Alloy selection should align with both mechanical needs and aesthetic requirements.
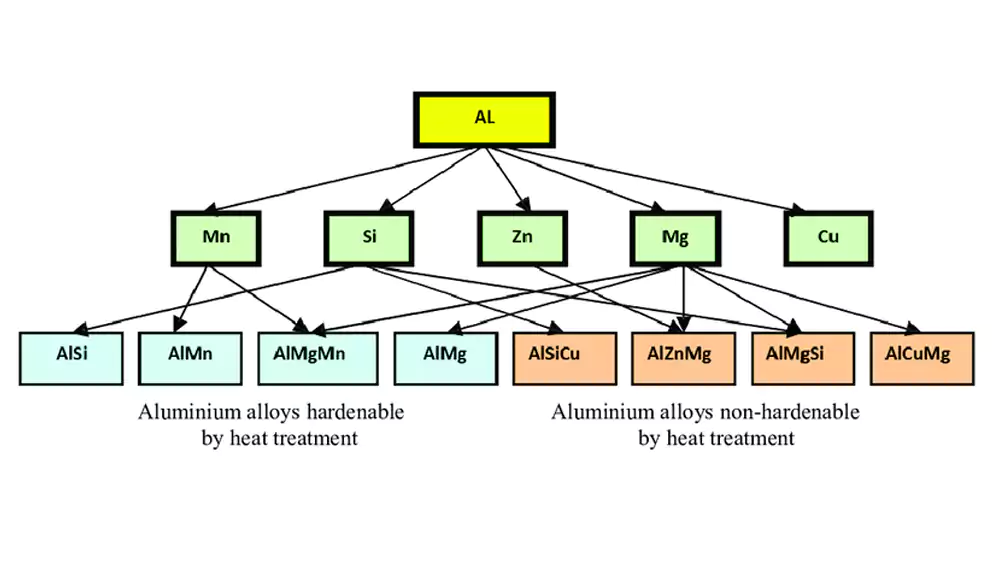
Alloy Element Effects on Anodizing
The presence of alloying elements creates distinct challenges and opportunities:
- Silicon: Creates dark gray to black appearance, common in cast alloys
- Copper: Can cause darker finishes and reduced corrosion resistance
- Magnesium: Generally improves corrosion resistance after anodizing
- Zinc: May produce yellowish tones in the anodized layer
- Manganese: Can cause brownish discoloration in thicker coatings
Practical Alloy Selection Guidelines
For most applications, I recommend 6061 aluminum5 - it offers the best balance of mechanical properties, machinability, and consistent anodizing results. For high-strength applications, 7075 works well but may show some color variation. When appearance is critical, 5052 or 6063 provide the most uniform finishes. Always discuss anodizing requirements with your machine shop during material selection.
Main Types of Anodizing
Understanding the different anodizing types helps match the process to your specific performance requirements and budget constraints.
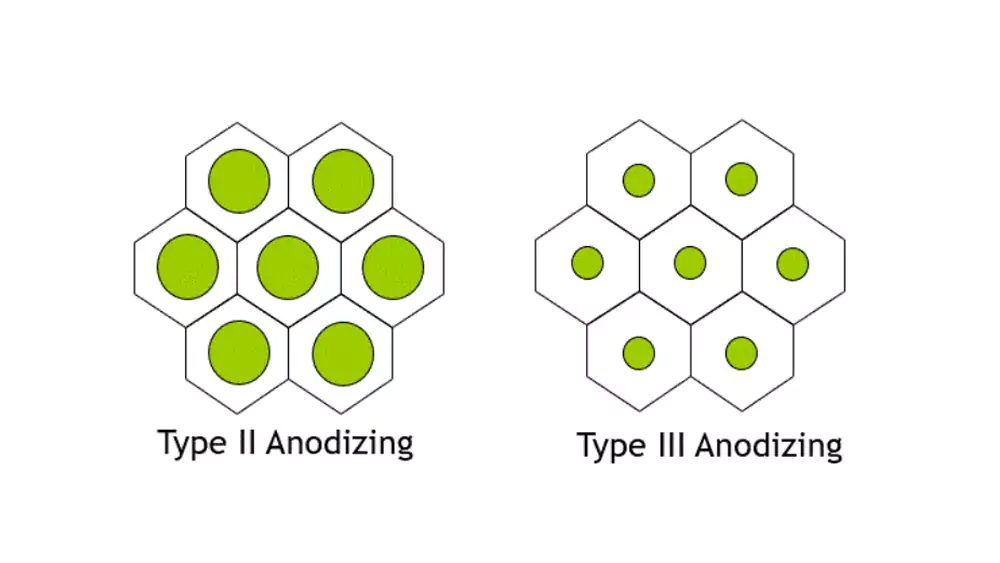
The three main anodizing types are Type I (chromic acid), Type II (sulfuric acid), and Type III (hard coat). Type II is most common for decorative applications, while Type III provides maximum wear resistance for demanding environments.
Type I - Chromic Acid Anodizing
Characteristics and Applications
Chromic acid anodizing produces the thinnest coatings (0.00002-0.00007 inches) with excellent corrosion protection. It's particularly valuable for complex parts with tight tolerances since it adds minimal dimension. The process is commonly specified in aerospace applications (MIL-A-8625 Type I) where fatigue resistance is critical.
Limitations and Considerations
The use of chromic acid presents environmental and safety challenges, requiring specialized waste treatment. The thin coatings provide limited wear resistance compared to other types, and color options are limited to light shades due to the coating's thinness.
Type II - Sulfuric Acid Anodizing
Process Advantages and Versatility
This is our most requested anodizing type, offering good corrosion protection and excellent color options at reasonable cost. Coating thickness typically ranges from 0.0002-0.0007 inches, providing a balance of properties suitable for most applications. The process accepts a wide range of organic and inorganic dyes.
Commercial Applications
Type II dominates consumer products, architectural applications, and general industrial components. The combination of appearance options, good durability, and reasonable cost makes it the default choice for many applications. It meets MIL-A-8625 Type II specifications for military applications.
Type III - Hard Coat Anodizing
Extreme Performance Characteristics
Hard anodizing builds much thicker coatings (0.002-0.004 inches) that approach the hardness of tool steel. The process uses lower temperatures and higher current densities to create extremely dense, wear-resistant surfaces. The resulting coating provides excellent abrasion resistance and thermal insulation.
Specialized Industrial Applications
We specify Type III for military equipment, hydraulic components, firearm parts, and anywhere extreme wear resistance is needed. The process can reduce part dimensions significantly, so machining allowances must be considered. The natural color is dark gray to black, with limited options for coloring.
Key Process Parameters & Film Properties
Controlling anodizing parameters allows engineers to precisely tailor surface properties for specific applications.
Key anodizing parameters include electrolyte concentration, temperature, current density, and process time. These factors directly control coating thickness, hardness, porosity, and corrosion resistance. Proper parameter selection ensures consistent, predictable results.
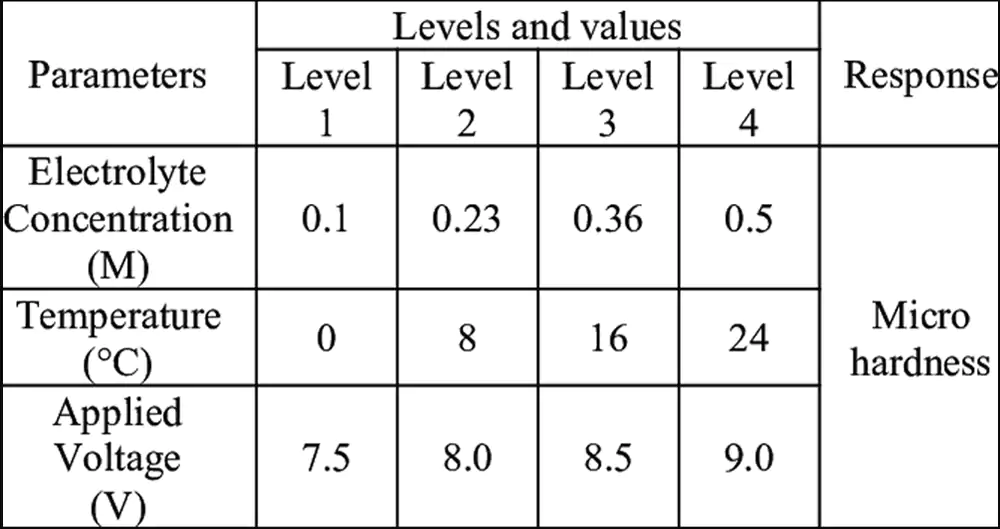
Parameter Control for Consistent Results
Let me share practical parameter guidelines from our shop. For Type II decorative anodizing, we typically run at 12-18 volts in 15-20% sulfuric acid at 70°F. This gives us about 0.0002-0.0007 inches thickness in 30-45 minutes. For Type III hard coat, we use lower temperatures (32-40°F) and higher current densities (24-36 volts) to build 0.002-0.004 inch thick coatings in 60-90 minutes.
Film Property Relationships
The relationship between process parameters and final properties follows predictable patterns. Higher current densities increase growth rates but may reduce coating density. Lower temperatures produce harder, denser coatings with reduced porosity. Longer processing times increase thickness linearly but may reduce coating quality if pushed beyond optimal ranges. We document every parameter for traceability, essential for medical and aerospace customers.
Case Study: Industrial Robotics Component Anodizing
We recently anodized aluminum housings for an industrial robotics company that demonstrates the practical application of these principles.
The Challenge: 500 units of 6061 aluminum sensor housings needed protection against coolants and repetitive handling while maintaining precise dimensions and a corporate blue color across multiple production batches.
Our Solution: Type II sulfuric acid anodizing with organic dye and hot water sealing for maximum durability and color consistency.
| Parameter | Specification | Measured Results | Test Method |
|---|---|---|---|
| Coating Thickness | 0.0003 ±0.0001 inches | 0.00032 inches average | Eddy Current |
| Salt Spray Resistance | 500 hours minimum | 750 hours no corrosion | ASTM B117 |
| Color Consistency | ΔE < 2.0 | ΔE 1.2 average | Spectrophotometer |
| Dimensional Change | ±0.0002 inches | +0.00015 inches | CMM Measurement |
| Adhesion | Tape test pass | No peeling | ASTM D3359 |
| Surface Hardness | >400 HV | 450 HV | Microhardness |
The anodizing process increased production costs by 15% but extended component lifespan by 300%, providing excellent ROI. The consistent blue finish also improved the customer's brand recognition across their product line while ensuring reliable performance in harsh manufacturing environments.
Advantages and Disadvantages of Anodizing
Understanding the complete picture of anodizing's strengths and limitations ensures proper application in your designs.
Anodizing offers excellent wear resistance, permanent coloring, and environmental safety, but has limitations with non-aluminum metals, color matching challenges, and potential for electrical conductivity issues. Understanding these trade-offs ensures proper application selection.

Significant Advantages for Engineering Applications
The benefits make anodizing our top recommendation for aluminum components in many scenarios. The hardness achieved, particularly with Type III, rivals many tool steels, making it ideal for high-wear applications. Unlike paint, the color won't chip or peel because it's integral to the surface. The process is also environmentally friendly compared to plating, using water-based solutions with lower toxicity.
Practical Limitations and Workarounds
However, anodizing only works reliably with aluminum, titanium, and magnesium. Color matching between batches can be challenging, particularly with lighter shades. The coating isn't electrically conductive, which can be problematic for grounding applications. Cost is moderate - more expensive than powder coating but cheaper than most plating processes. Part size is limited by tank dimensions, and rack marks may be visible if not strategically placed.
Anodizing vs. Powder Coating vs. Electroplating: How to Choose?
Selecting the optimal surface treatment requires careful evaluation of your specific performance requirements, budget, and design constraints.
Choose anodizing for hardness and dimensional stability, powder coating for color variety and impact resistance, or electroplating for electrical conductivity and unique metallic appearances. Each process serves different design needs with distinct cost and performance trade-offs.

Comprehensive Process Comparison
| Criteria | Anodizing | Powder Coating | Electroplating |
|---|---|---|---|
| Material Compatibility | Aluminum, Titanium, Magnesium | Most metals | Most metals |
| Wear Resistance | Excellent | Good | Fair to Good |
| Corrosion Protection | Very Good | Excellent | Good |
| Color Options | Limited | Unlimited | Metallic finishes only |
| Electrical Properties | Insulating | Insulating | Conductive |
| Dimensional Impact | Small increase | Significant increase | Small increase |
| Environmental Impact | Low-medium | Low | High |
| Relative Cost | Medium | Low | High |
Decision Framework for Engineers
I help customers choose between these processes using a simple framework. Start with material compatibility - anodizing only works with specific metals. Then consider functional requirements: need electrical conductivity? Eliminate anodizing. Require extreme hardness? Anodizing wins. Next, evaluate appearance needs: specific color matching favors powder coating, while metallic appearance suggests plating. Finally, consider production volume and cost targets.
Application-Specific Recommendations
For automotive components exposed to road salts, powder coating often provides the best corrosion protection. For electronic enclosures needing EMI shielding, electroplating offers conductivity. For mechanical components requiring wear resistance with minimal dimensional change, anodizing is typically optimal. Always prototype with your intended surface treatment to validate performance before full production.
Conclusion
Anodizing transforms aluminum surfaces into durable, functional finishes that outperform applied coatings in many applications. Understanding the process fundamentals ensures successful implementation in your designs and prevents costly manufacturing errors.
-
Exploring aluminum oxide's properties can provide insights into its versatility in manufacturing and technology. ↩
-
Exploring quality verification methods can enhance your knowledge of maintaining high standards in production. ↩
-
This article is about electric current density. For the quantum concept. ↩
-
Learn more about how to prevent rust on metal castings. ↩
-
Explore the advantages of 6061 aluminum, known for its excellent anodizing results and mechanical properties. ↩
